Charles' Law
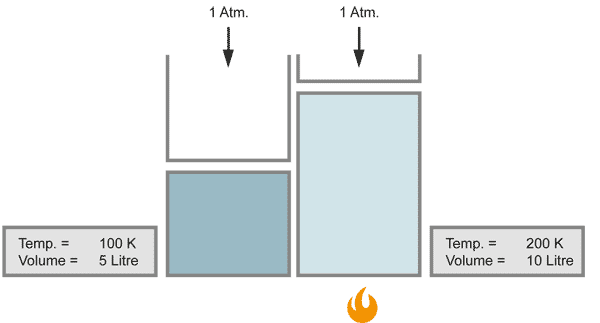
The volume of a gas changes with temperature if the pressure is kept constant. When the gas becomes colder, the volume decreases. As it becomes warmer, the gas expands.
T in Kelvin (see scale of absolute temperature below).
When the temperature doubles, the volume increases similarily by the factor of 2, provided that the pressure stays constant.
with equal numbers of molecules and constant pressure
With a closer look at Charles` law, one can find a more detailed relationship between temperature and volume, known as the law of Gay-Lussac: